CLEARLine®
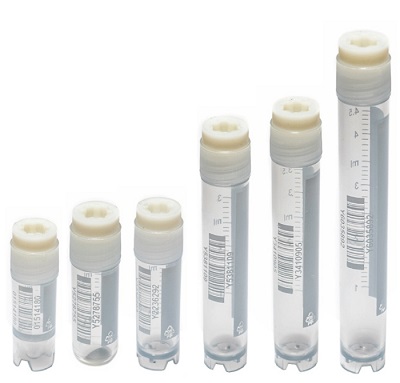
Biosigma s.r.l. and STIRILAB s.r.o. have created and use mark CLEARLine® for certification of cleanliness of our products as cryotubes and micro cryotubes to fulfill the highest requirements of medical, pharmaceutical and food industries in a way comparable to molecular biology and cell technology.
CLEARLine® mark certifies:

Cryotubes and „snap cap“ micro tubes as not containing:
human DNA/DNase/RNase/ATP/Pyrogenes/PCR inhibitors

Micro tubes with an external cap as not containg:
DNase/RNase/Pyrogenes
Product cleanliness is secured by the automated sophisticated production process, which protects product against any contamination by biological materials using CLEAN ROOM class ISO 7 (UNI EN ISO 14644-1) class 10.000 (US FED STD 209E).
CLEARLINE products are produced using primary material for medical purposes according to UPS CLASS VI, certificate ISO 10993, which is non-cytotoxic, non-hemolytic, not containing heavy metals and ingredients of animal origin.
Certificate is issued for each batch by an independent external laboratory and it is possible to download it from website www.biosigma.com/certificate providing batch number.

 Slovenčina
Slovenčina